How to Determine the Most Acidic Proton
The lone pair on b is more stable due to resonance than the lone pair on a. Who are the experts.
How To Identify The Most Acidic Proton
Its conjugate base is the weakest base here and.
. X is an alkyl proton adjacent to a carbonyl. There are three general methods to estimate the acidity of a proton. 2 Identify which functional group the proton is a part of like.
Show transcribed image text Expert Answer. P is an amide proton. The molecule is Vitamin C ascorbic acid and the most acidic proton is the lower left.
Compare the O H bonds in Vitamin C ascorbic acid and decide which one is the most acidic. Identify the most acidic proton in each compound and suggest a reason for the trend in. HN HN ОН о ОН.
You can delocalize much more including the CC double bond and the ester group if you deprotonate there. Identifying Acidic Protons 1. To summarize whenever you need to determine the more acidic proton visualize or better draw the conjugate bases and determine which one is the more stable base on the ARIO factors.
Removal of either of these H s at hydroxyl group A or B does not give a resonance stabilized anion. The lone pair on nitrogen b is the most basic. This means the most acidic proton in this molecule is the on the terminal alkyne sp C-H.
Alkane alcohol carboxylic acid protonated. Therefore p should be the most acidic. An alkene proton has a pKa in the 40s and the aldehyde hydrogen is even higher.
How to find most acidic hydrogen Most acidic hydrogen example 1 picking the most acidic hydrogen in a molecule Ch 2 OHV Identifying the most acidic proton in a molecule. Hydrogens attached to a positively charged nitrogen oxygen or sulfur are acidic. Scan and rank sounds simple but it conceals several difficulties that are elaborated below.
However when acting as acids only the most acidic proton will participate in the acid-base reaction. S c h e m e 1. We review their content and use your feedback to keep the quality high.
Z is an amine proton. The high electronegativity of. Recall that in carbonyl chemistry removal of the alpha hydrogen yields the resonance stabilized enolate.
You can explain the acidity of vitamin C by regarding it as. The most acidic proton in ascorbic acid is the one whose conjugate base is most resonance stabilized. Thus the final acidic order of the discussed compounds is.
HI with a pK a of about -9 is one the strongest acids known. Only two protons are acidic and they are A and B. As evidenced by the pK a.
Acidic protons are usually bound to O or N. Carboxylic acid Phenol Water Alcohol. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Diphenylmethane is significantly more acidic than benzene and triphenylmethane is more acidic. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Most molecules contain different types of protons attached hydrogens.
DO NOT FORGET TO SUBSCRIBEThis video illustrates how to determine the most acidic proton as well as how to determine which molecule is more acidic based on. Y is an alkyl proton para to a carbonyl. On comparing the acidity of carboxylic acid phenol and alcohol Carboxylic acids are stronger acids than corresponding alcohols and even phenols because it loses its proton to form a stable conjugate base.
Answer 1 of 19. Hydrogens directly attached to very electronegative atoms such as oxygen sulphur and the halogens carry a. The conjugate acid of a is an alcohol which has a pKa of 16.
Therefore b is the weaker base and a is the stronger base. Therefore it is important to be able to identify the most acidic proton in. Experts are tested by Chegg as specialists in their subject area.
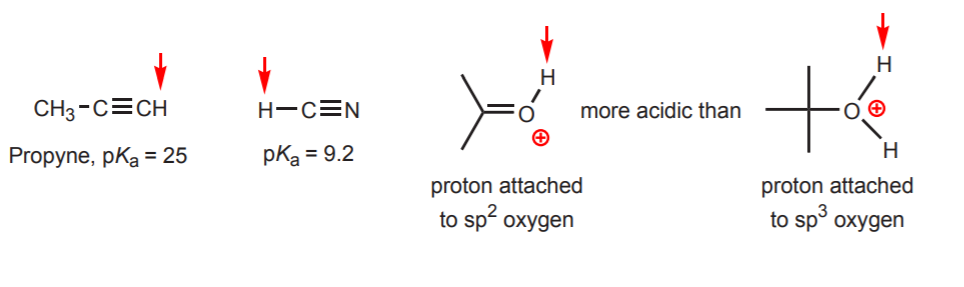
Diphenylmethane is significantly more acidic than benzene and triphenylmethane is. Sp3 hybridized carbons are less acidic than sp2 which is less acidic than sp because the greater the s character of the bond the more stable is the negative charge of the carbanion. The hydrocarbons are generally considered very weak acids but among them the alkynes with a pKa 25 are quite acidic.
More importantly to the study of biological organic chemistry this trend tells us that thiols are more acidic than alcohols. How do you determine which proton is the most acidic in this molecule. 1 Use the table of common acids that we learned in class and is in your book Table 31 inside front cover of the.
Therefore the first step is to look for all OH and NH bonds. This video gives u a key concept to find most acidic hydrogen in a moleculeTo support me in my journey you can donate Paytm 7870993388A small amount of. Sp3 hybridized carbons are more acidic than sp2 if the corresponding carbanions negative charge is distributed in a pi-system such as the allylic proton D.
Not a chance. Scan a molecule for known acidic functional groups. The most acidic functional group usually is holding the most acidic H in the entire molecule.
The pK a of the thiol group on the cysteine side chain for example is approximately 83 while the pK a for the hydroxl on the serine side chain is on the.

How To Find The Most Acidic H In A Molecule Youtube
11 10 Identifying Acidic Protons Chemistry Libretexts
Ch 2 Ohv Identifying The Most Acidic Proton In A Molecule Youtube
No comments for "How to Determine the Most Acidic Proton"
Post a Comment